The evidence behind the autoimmune protocol diet (and how to try AIP)
For a long time, I thought I was just stuck with eczema. I’d had the itchy, scaly skin rash for nineteen-some years, and my allergist and dermatologist just kept giving me stronger and stronger steroid creams. In 2013, my sister persuaded me to try a paleo-type diet. My eczema improved dramatically but still seemed to linger around. It was only when I found the autoimmune protocol (AIP) diet that I was able to experience completely clear skin for the first time in my life.
I’m not the only one, though. Hundreds, if not thousands, of other people have used the AIP diet to put their autoimmune disease (or similar conditions) into remission. But what’s the mechanism? And do we have any evidence as to why cutting out certain foods would actually work? In this article, I’ll cut through the hearsay and discuss the real science behind AIP.
This article is for anyone who is:
- New to AIP and wants to learn more about it
- Considering trying AIP, but not fully convinced why they should
- Currently on AIP, and just wanting to understand more of the science behind it
- Currently on AIP, but struggling with friends or doctors that think this is just another “fad” diet
To begin, I’ll need to give some brief background on what goes wrong in autoimmune disease. Genetic predisposition accounts for about one-third of your risk for developing autoimmune disease. The other two-thirds come from your diet, environment, and lifestyle. Given that our gut is home to 70% of our immune cells and the first line of defense against the outside environment, it’s a good place to look for answers.
The gut microbiome and dysbiosis
Our gut is home to trillions of microbes that play crucial roles in our health. They aid in digestion, synthesize vitamins, and help educate the immune system.1 Our commensal, or “good” microbes, also help to outcompete pathogenic, or “bad” microbes. Everyone has some potentially pathogenic microbes in their gut, but to put it simply, we need enough of the “good” microbes to keep the “bad” microbes in check. If this delicate balance is disrupted, we call this gut dysbiosis.
Numerous studies have shown that autoimmune disease is associated with gut dysbiosis, or an altered gut microbiome.2 For instance, patients with psoriatic arthritis have been shown to have reduced bacterial diversity. Patients with multiple sclerosis have reduced levels of Butyricomas, a genus of bacteria known to produce the beneficial gut metabolite butyrate.3 Patients with lupus and Type 1 diabetes have lower levels of bacteria in the phylum Firmicutes and more bacteria in the phylum Bacteroidetes.4,5
The gut barrier and oral tolerance
The gut is a long, hollow tube that is lined with tiny epithelial cells. These epithelial cells form a single layer about the width of a human hair. The epithelial cells are responsible for absorbing nutrients from food while maintaining a tight barrier to keep large dietary proteins, microbes, and toxins out.
Besides epithelial cells, both the small and large intestine also have gut-associated lymphoid tissue, or GALT for short. This highly innervated immune tissue lies throughout the small and large intestine and covers an area of approximately 260-300 square meters. About 70% of all immune cells lie in the GALT.
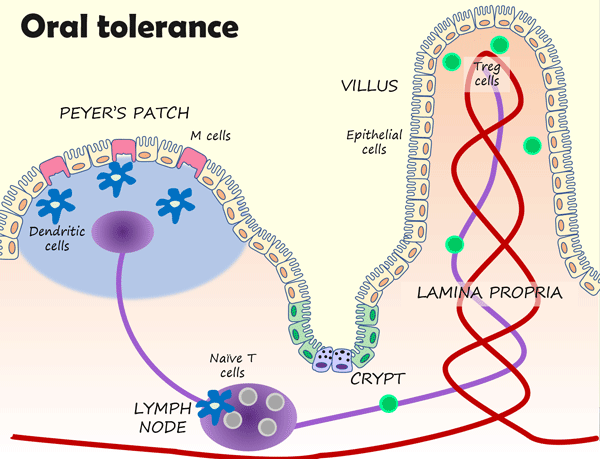
Many of these immune cells are found within dome-like structures called Peyer’s patches. Peyer’s patches contain a mixture of immune cells, including lymphocytes (B and T cells), macrophages, and dendritic cells. Macrophages and dendritic cells constantly “sample” bacteria and dietary antigens in the gut lumen, and then “present” their findings to naive T cells in lymph nodes. In a calm gut state, this will generally cause T cells to differentiate into regulatory T cells (Tregs). Tregs migrate into the tissue beneath the epithelium (the lamina propria) to release anti-inflammatory cytokines, suppress immune responses, and promote oral tolerance.
Intestinal permeability and food hypersensitivities
A breakdown of the gut barrier (intestinal permeability, or “leaky gut”) may lead to the influx of bacteria, bacterial cell wall components, and dietary proteins into the submucosa, the region just beneath the epithelial cells. This area is rich in immune cells which will sense the invasion, secrete pro-inflammatory cytokines, and recruit more immune cells to the scene.
Macrophages and dendritic cells will chew up invading dietary proteins and “present” their findings to T cells. This time, the inflammatory microenvironment will cause them to differentiate into effector T cells, which will help curb the invasion but also prime the immune system for future attacks.
Some of these substances may also pass through the gut-vascular barrier into the bloodstream, where they can trigger a systemic immune response.6 The immune system will form antibodies against the microbial toxins and dietary antigens, which can continue to circulate in the blood for many months. Unfortunately, many of these antibodies that are formed to react with dietary proteins can also react with our own body tissues7 – this is the basis for autoimmunity.
Intestinal permeability is so closely associated with food allergies and food hypersensitivities,8 that some leading researchers think that intestinal permeability is a prerequisite for developing autoimmune disease.9 In other words, you can have a genetic predisposition to autoimmune disease, but you won’t actually develop it until you have something that triggers a breakdown of the gut barrier.10 Increasingly, this paradigm is being more widely accepted by the medical community.11
What triggers gut dysbiosis and barrier breakdown?
A number of things can influence the composition of the gut microbiota and the health of the gut barrier. These include stress, medications, antibiotics, environmental toxins, exercise, and sleep. Diet also plays a major role, since we are constantly exposing our gut to different compounds in foods. If our gut mucosal immune system is sensitized to a food, the inflammatory cytokines produced in response to it can further damage the gut barrier.12
It follows that if we can identify the foods that are contributing to gut dysbiosis, intestinal permeability, and abnormal mucosal immune responses — and remove them from the diet for a period of time – then we can quiet inflammation and restore gut barrier integrity. This is the overall basis for the autoimmune protocol diet.
The overarching principles of AIP:
The autoimmune protocol diet is an anti-inflammatory, nutrient-dense elimination diet. It initially eliminates grains, legumes, dairy, nightshades, eggs, alcohol, nuts, seeds (including coffee, cocoa, and seed-based spices), refined sugar, and food additives. The basic rationale is avoidance of any foods that might trigger intestinal inflammation, promote microbial dysbiosis, or that are highly immunogenic and represent common sensitivities.13 Below, I’ve provided a more detailed rationale for the removal of each individual food group:
Grains and legumes: Gliadin, a protein in gluten and wheat, has been shown to cause intestinal permeability in non-celiac intestinal cell lines14,15. Gliadin and wheat germ agglutinin also cause inflammation in immune cells in vitro.16 Grains and legumes are also high in saponins, and even low concentrations of saponins may act as an adjuvant to amplify immune responses and increase gut permeability.17–19 A grain-free diet has been shown to reduce inflammation in animal models.20
Nightshades: The nightshade family of plants include tomatoes, sweet and hot peppers, potatoes, eggplants, and chili-based spices like cayenne and paprika. Nightshades are particularly problematic for those with autoimmune disease. Like grains and legumes, they can contain high levels of saponins. Some nightshades, like chili peppers and the spices cayenne and paprika, also contain capsaicin, a compound has been shown to increase intestinal permeability.21
Dairy: While dairy can be a healthful addition to your diet if you tolerate it well, dairy is a common sensitivity in those with autoimmune disease. Even in those who don’t have lactose intolerance, proteins like casein can often trigger a gut immune response and antibody formation.22
Eggs: Eggs are one of the most allergenic foods. The five most allergenic proteins in eggs are lysozyme, ovomucoid, ovalbumin, ovotransferrin, and ovomucin.23 Lysozyme is positively charged and may also enhance the allergenicity of other egg proteins by forming complexes with them and “shuttling” them across the epithelium.
Alcohol: Alcohol consumption, even as little as one drink, has been shown to increase intestinal permeability.24 Alcohol consumption also promotes microbial dysbiosis, stresses the liver, and can contribute to SIBO.25
Nuts and seeds: The rationale behind eliminating nuts and seeds is a little less clear. However, tree nuts are among the most common allergens and food sensitivities.26 Nuts and seeds also have several antinutrients, though few studies have assessed their effects on intestinal permeability. Of all the foods eliminated on AIP, properly prepared seeds are the most likely to be successfully reintroduced. However, it’s still a good idea to eliminate them for 30 days to see how your body responds.
Refined sugar and food additives: Several common food additives, including emulsifiers and refined sugar, have been shown to increase intestinal permeability.27 Refined sugar is well established to feed pathogenic microbes in the gastrointestinal tract,28 and non-nutritive sweeteners have been shown to cause gut microbial dysbiosis and cause glucose intolerance.29
The diet also emphasizes consumption of fresh, nutrient-dense, fiber-rich, and fermented foods, which may help promote gut healing and oral tolerance to foods.
- Organ meats
- Bone broth
- Seafood
- Vegetables
- Fermented foods
The overall goal is to promote a healthy gut microbiota, allow the gut barrier to heal, and help regulate the immune system. To this end, the autoimmune protocol also emphasizes the importance of exercise, sleep, and stress management.
Reintroduction of foods
Elimination of these foods (with the exception of refined sugar and food additives) is not intended to be long-term. Many of these foods are perfectly healthy in the context of a healthy gut microbiota, gut barrier, and immune system.
After a period of time long enough to see a reduction in symptoms, gradual reintroduction of these foods will allow you to broaden your diet while identifying foods that might be contributing to your symptoms.
For instance, I know that I need to avoid gluten, corn, soy, and nuts completely, but I have been able to successfully reintroduce white rice, potatoes, bell peppers, nightshade spices, eggs, and seeds into my diet without compromising my skin or overall health. With other foods, like tomatoes and dairy, I can eat them on occasion without much issue, but feel best if I don’t consume them regularly. AIP is not about arbitrary restriction – it’s about finding out which foods make you feel your best.
Scientific studies on AIP:
So, what about AIP in the medical literature? Funding for studies that use real food is hard to come by. However, one study published in 2017 looked at the effects of the AIP diet on inflammatory bowel disease (IBD).13
Dr. Gauree Konijeti, a Gastroenterologist at Scripps Research Institute had a patient who happened to try AIP and saw remarkable clinical improvement in his ulcerative colitis. A repeat endoscopy on the patient showed that his ulcers were gone! Dr. Konijeti was impressed, and she decided that the diet was worth testing in a clinical trial.
For the study, she and her team enrolled 15 patients that had been diagnosed with IBD for an average of 19 years. With the help of Angie Alt’s SAD to AIP in six program, they counseled the participants through a six-week, phased elimination program that slowly transitioned them onto AIP, cutting out a few food groups each week. The participants then remained on full AIP for five more weeks of observation. The results were pretty remarkable:
“Clinical remission was achieved by week 6 by 11/15 (73%) of study participants, and all 11 maintained clinical remission during the maintenance phase of the study.”
To put this in perspective, the best drug treatments for IBD have about a 50% response rate.30 In other words, improvement from six weeks of AIP rivaled most drug therapies for IBD – without any of the unpleasant side effects.
To date, this is the only study published on the autoimmune protocol diet. However, based on all of the research surrounding the gut’s role in autoimmune disease, the evidence for the removal of each food group, and the results from this pilot clinical trial in IBD patients, there is every reason to believe that AIP might be an effective adjuvant treatment for many people with autoimmune disease and related conditions.
As part of my dissertation research, I’m planning to perform a similar trial of AIP in 20 patients with atopic dermatitis. In addition to assessing changes in skin symptoms, we will also be tracking how the diet changes the gut microbiome and intestinal permeability! I’m excited to learn more about how AIP works on the molecular level.
AIP quick start guide
So, how to get started? The simple answer is to remove the foods listed above and focus on nutrient-dense foods like meat, broth, vegetables and fermented foods. For more detailed instructions, here are a few of my favorite resources for getting started with AIP:
The Autoimmune Protocol – Sarah Ballantyne’s quick start guide to AIP
“AIP for you and me” – an AIP Facebook support group
The Paleo Approach – Sarah Ballantyne’s book (and matching cookbook)
SAD to AIP in six – Angie Alt’s program used in the AIP for IBD study
And here are a few of my favorite AIP-friendly recipes:
That’s all for now! If you enjoyed this article, be sure to subscribe. You can also click here to learn more about working with me one-on-one.
Sources:
- Flint, H. J., Scott, K. P., Louis, P. & Duncan, S. H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589 (2012).
- Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M. & Owen, L. J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191 (2015).
- Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016).
- He, Z., Shao, T., Li, H., Xie, Z. & Wen, C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 8, 64 (2016).
- Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5, 82–91 (2011).
- Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
- Vojdani, A. & Tarash, I. Cross-Reaction between Gliadin and Different Food and Tissue Antigens. Food Nutr. Sci. 04, 20 (2013).
- Ventura, M. T. et al. Intestinal permeability in patients with adverse reactions to food. Dig. Liver Dis. 38, 732–736 (2006).
- Visser Jeroen, Rozing Jan, Sapone Anna, Lammers Karen & Fasano Alessio. Tight Junctions, Intestinal Permeability, and Autoimmunity. Ann. N. Y. Acad. Sci. 1165, 195–205 (2009).
- König, J. et al. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 7, e196 (2016).
- Vaarala, O., Atkinson, M. A. & Neu, J. The “Perfect Storm” for Type 1 Diabetes: The Complex Interplay Between Intestinal Microbiota, Gut Permeability, and Mucosal Immunity. Diabetes 57, 2555–2562 (2008).
- Ma, T. Y. et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G367-376 (2004).
- Konijeti, G. G. et al. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 23, 2054–2060 (2017).
- Lammers, K. M. et al. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 135, 194-204.e3 (2008).
- Drago, S. et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 41, 408–419 (2006).
- Thomas, K. E., Sapone, A., Fasano, A. & Vogel, S. N. Gliadin Stimulation of Murine Macrophage Inflammatory Gene Expression and Intestinal Permeability Are MyD88-Dependent: Role of the Innate Immune Response in Celiac Disease. J. Immunol. 176, 2512–2521 (2006).
- Francis, G., Kerem, Z., Makkar, H. P. S. & Becker, K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 88, 587–605 (2002).
- Gee, J. M. et al. Effects of saponins and glycoalkaloids on the permeability and viability of mammalian intestinal cells and on the integrity of tissue preparations in vitro. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 10, 117–128 (1996).
- Patel, B. et al. Potato glycoalkaloids adversely affect intestinal permeability and aggravate inflammatory bowel disease. Inflamm. Bowel Dis. 8, 340–346 (2002).
- Jönsson, T. et al. A Paleolithic diet confers higher insulin sensitivity, lower C-reactive protein and lower blood pressure than a cereal-based diet in domestic pigs. Nutr. Metab. 3, 39 (2006).
- Jensen-Jarolim, E. et al. Hot spices influence permeability of human intestinal epithelial monolayers. J. Nutr. 128, 577–581 (1998).
- Monetini, L. et al. Antibodies to Bovine Beta-Casein in Diabetes and Other Autoimmune Diseases. Horm. Metab. Res. 34, 455–459 (2002).
- Heine, R. G., Laske, N. & Hill, D. J. The diagnosis and management of egg allergy. Curr. Allergy Asthma Rep. 6, 145–152 (2006).
- Purohit, V. et al. Alcohol, Intestinal Bacterial Growth, Intestinal Permeability to Endotoxin, and Medical Consequences. Alcohol Fayettev. N 42, 349–361 (2008).
- Patel, S. et al. Alcohol and the Intestine. Biomolecules 5, 2573–2588 (2015).
- Roux, K. H., Teuber, S. S. & Sathe, S. K. Tree Nut Allergens. Int. Arch. Allergy Immunol. 131, 234–244 (2003).
- Lerner, A. & Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 14, 479–489 (2015).
- Graf, D. et al. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 26, 26164 (2015).
- Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
- Martínez-Montiel, M. et al. Pharmacologic therapy for inflammatory bowel disease refractory to steroids. Clin. Exp. Gastroenterol. 8, 257–269 (2015).
The evidence behind the autoimmune protocol diet (and how to try AIP)
For a long time, I thought I was just stuck with eczema. I’d had the itchy, scaly skin rash for nineteen-some years, and my allergist and dermatologist just kept giving me stronger and stronger steroid creams. In 2013, my sister persuaded me to try a paleo-type diet. My eczema improved dramatically but still seemed to linger around. It was only when I found the autoimmune protocol (AIP) diet that I was able to experience completely clear skin for the first time in my life.
I’m not the only one, though. Hundreds, if not thousands, of other people have used the AIP diet to put their autoimmune disease (or similar conditions) into remission. But what’s the mechanism? And do we have any evidence as to why cutting out certain foods would actually work? In this article, I’ll cut through the hearsay and discuss the real science behind AIP.
This article is for anyone who is:
- New to AIP and wants to learn more about it
- Considering trying AIP, but not fully convinced why they should
- Currently on AIP, and just wanting to understand more of the science behind it
- Currently on AIP, but struggling with friends or doctors that think this is just another “fad” diet
To begin, I’ll need to give some brief background on what goes wrong in autoimmune disease. Genetic predisposition accounts for about one-third of your risk for developing autoimmune disease. The other two-thirds come from your diet, environment, and lifestyle. Given that our gut is home to 70% of our immune cells and the first line of defense against the outside environment, it’s a good place to look for answers.
The gut microbiome and dysbiosis
Our gut is home to trillions of microbes that play crucial roles in our health. They aid in digestion, synthesize vitamins, and help educate the immune system.1 Our commensal, or “good” microbes, also help to outcompete pathogenic, or “bad” microbes. Everyone has some potentially pathogenic microbes in their gut, but to put it simply, we need enough of the “good” microbes to keep the “bad” microbes in check. If this delicate balance is disrupted, we call this gut dysbiosis.
Numerous studies have shown that autoimmune disease is associated with gut dysbiosis, or an altered gut microbiome.2 For instance, patients with psoriatic arthritis have been shown to have reduced bacterial diversity. Patients with multiple sclerosis have reduced levels of Butyricomas, a genus of bacteria known to produce the beneficial gut metabolite butyrate.3 Patients with lupus and Type 1 diabetes have lower levels of bacteria in the phylum Firmicutes and more bacteria in the phylum Bacteroidetes.4,5
The gut barrier and oral tolerance
The gut is a long, hollow tube that is lined with tiny epithelial cells. These epithelial cells form a single layer about the width of a human hair. The epithelial cells are responsible for absorbing nutrients from food while maintaining a tight barrier to keep large dietary proteins, microbes, and toxins out.
Besides epithelial cells, both the small and large intestine also have gut-associated lymphoid tissue, or GALT for short. This highly innervated immune tissue lies throughout the small and large intestine and covers an area of approximately 260-300 square meters. About 70% of all immune cells lie in the GALT.
Many of these immune cells are found within dome-like structures called Peyer’s patches. Peyer’s patches contain a mixture of immune cells, including lymphocytes (B and T cells), macrophages, and dendritic cells. Macrophages and dendritic cells constantly “sample” bacteria and dietary antigens in the gut lumen, and then “present” their findings to naive T cells in lymph nodes. In a calm gut state, this will generally cause T cells to differentiate into regulatory T cells (Tregs). Tregs migrate into the tissue beneath the epithelium (the lamina propria) to release anti-inflammatory cytokines, suppress immune responses, and promote oral tolerance.
Intestinal permeability and food hypersensitivities
A breakdown of the gut barrier (intestinal permeability, or “leaky gut”) may lead to the influx of bacteria, bacterial cell wall components, and dietary proteins into the submucosa, the region just beneath the epithelial cells. This area is rich in immune cells which will sense the invasion, secrete pro-inflammatory cytokines, and recruit more immune cells to the scene.
Macrophages and dendritic cells will chew up invading dietary proteins and “present” their findings to T cells. This time, the inflammatory microenvironment will cause them to differentiate into effector T cells, which will help curb the invasion but also prime the immune system for future attacks.
Some of these substances may also pass through the gut-vascular barrier into the bloodstream, where they can trigger a systemic immune response.6 The immune system will form antibodies against the microbial toxins and dietary antigens, which can continue to circulate in the blood for many months. Unfortunately, many of these antibodies that are formed to react with dietary proteins can also react with our own body tissues7 – this is the basis for autoimmunity.
Intestinal permeability is so closely associated with food allergies and food hypersensitivities,8 that some leading researchers think that intestinal permeability is a prerequisite for developing autoimmune disease.9 In other words, you can have a genetic predisposition to autoimmune disease, but you won’t actually develop it until you have something that triggers a breakdown of the gut barrier.10 Increasingly, this paradigm is being more widely accepted by the medical community.11
What triggers gut dysbiosis and barrier breakdown?
A number of things can influence the composition of the gut microbiota and the health of the gut barrier. These include stress, medications, antibiotics, environmental toxins, exercise, and sleep. Diet also plays a major role, since we are constantly exposing our gut to different compounds in foods. If our gut mucosal immune system is sensitized to a food, the inflammatory cytokines produced in response to it can further damage the gut barrier.12
It follows that if we can identify the foods that are contributing to gut dysbiosis, intestinal permeability, and abnormal mucosal immune responses — and remove them from the diet for a period of time – then we can quiet inflammation and restore gut barrier integrity. This is the overall basis for the autoimmune protocol diet.
The overarching principles of AIP:
The autoimmune protocol diet is an anti-inflammatory, nutrient-dense elimination diet. It initially eliminates grains, legumes, dairy, nightshades, eggs, alcohol, nuts, seeds (including coffee, cocoa, and seed-based spices), refined sugar, and food additives. The basic rationale is avoidance of any foods that might trigger intestinal inflammation, promote microbial dysbiosis, or that are highly immunogenic and represent common sensitivities.13 Below, I’ve provided a more detailed rationale for the removal of each individual food group:
Grains and legumes: Gliadin, a protein in gluten and wheat, has been shown to cause intestinal permeability in non-celiac intestinal cell lines14,15. Gliadin and wheat germ agglutinin also cause inflammation in immune cells in vitro.16 Grains and legumes are also high in saponins, and even low concentrations of saponins may act as an adjuvant to amplify immune responses and increase gut permeability.17–19 A grain-free diet has been shown to reduce inflammation in animal models.20
Nightshades: The nightshade family of plants include tomatoes, sweet and hot peppers, potatoes, eggplants, and chili-based spices like cayenne and paprika. Nightshades are particularly problematic for those with autoimmune disease. Like grains and legumes, they can contain high levels of saponins. Some nightshades, like chili peppers and the spices cayenne and paprika, also contain capsaicin, a compound has been shown to increase intestinal permeability.21
Dairy: While dairy can be a healthful addition to your diet if you tolerate it well, dairy is a common sensitivity in those with autoimmune disease. Even in those who don’t have lactose intolerance, proteins like casein can often trigger a gut immune response and antibody formation.22
Eggs: Eggs are one of the most allergenic foods. The five most allergenic proteins in eggs are lysozyme, ovomucoid, ovalbumin, ovotransferrin, and ovomucin.23 Lysozyme is positively charged and may also enhance the allergenicity of other egg proteins by forming complexes with them and “shuttling” them across the epithelium.
Alcohol: Alcohol consumption, even as little as one drink, has been shown to increase intestinal permeability.24 Alcohol consumption also promotes microbial dysbiosis, stresses the liver, and can contribute to SIBO.25
Nuts and seeds: The rationale behind eliminating nuts and seeds is a little less clear. However, tree nuts are among the most common allergens and food sensitivities.26 Nuts and seeds also have several antinutrients, though few studies have assessed their effects on intestinal permeability. Of all the foods eliminated on AIP, properly prepared seeds are the most likely to be successfully reintroduced. However, it’s still a good idea to eliminate them for 30 days to see how your body responds.
Refined sugar and food additives: Several common food additives, including emulsifiers and refined sugar, have been shown to increase intestinal permeability.27 Refined sugar is well established to feed pathogenic microbes in the gastrointestinal tract,28 and non-nutritive sweeteners have been shown to cause gut microbial dysbiosis and cause glucose intolerance.29
The diet also emphasizes consumption of fresh, nutrient-dense, fiber-rich, and fermented foods, which may help promote gut healing and oral tolerance to foods.
- Organ meats
- Bone broth
- Seafood
- Vegetables
- Fermented foods
The overall goal is to promote a healthy gut microbiota, allow the gut barrier to heal, and help regulate the immune system. To this end, the autoimmune protocol also emphasizes the importance of exercise, sleep, and stress management.
Reintroduction of foods
Elimination of these foods (with the exception of refined sugar and food additives) is not intended to be long-term. Many of these foods are perfectly healthy in the context of a healthy gut microbiota, gut barrier, and immune system.
After a period of time long enough to see a reduction in symptoms, gradual reintroduction of these foods will allow you to broaden your diet while identifying foods that might be contributing to your symptoms.
For instance, I know that I need to avoid gluten, corn, soy, and nuts completely, but I have been able to successfully reintroduce white rice, potatoes, bell peppers, nightshade spices, eggs, and seeds into my diet without compromising my skin or overall health. With other foods, like tomatoes and dairy, I can eat them on occasion without much issue, but feel best if I don’t consume them regularly. AIP is not about arbitrary restriction – it’s about finding out which foods make you feel your best.
Scientific studies on AIP:
So, what about AIP in the medical literature? Funding for studies that use real food is hard to come by. However, one study published in 2017 looked at the effects of the AIP diet on inflammatory bowel disease (IBD).13
Dr. Gauree Konijeti, a Gastroenterologist at Scripps Research Institute had a patient who happened to try AIP and saw remarkable clinical improvement in his ulcerative colitis. A repeat endoscopy on the patient showed that his ulcers were gone! Dr. Konijeti was impressed, and she decided that the diet was worth testing in a clinical trial.
For the study, she and her team enrolled 15 patients that had been diagnosed with IBD for an average of 19 years. With the help of Angie Alt’s SAD to AIP in six program, they counseled the participants through a six-week, phased elimination program that slowly transitioned them onto AIP, cutting out a few food groups each week. The participants then remained on full AIP for five more weeks of observation. The results were pretty remarkable:
“Clinical remission was achieved by week 6 by 11/15 (73%) of study participants, and all 11 maintained clinical remission during the maintenance phase of the study.”
To put this in perspective, the best drug treatments for IBD have about a 50% response rate.30 In other words, improvement from six weeks of AIP rivaled most drug therapies for IBD – without any of the unpleasant side effects.
To date, this is the only study published on the autoimmune protocol diet. However, based on all of the research surrounding the gut’s role in autoimmune disease, the evidence for the removal of each food group, and the results from this pilot clinical trial in IBD patients, there is every reason to believe that AIP might be an effective adjuvant treatment for many people with autoimmune disease and related conditions.
As part of my dissertation research, I’m planning to perform a similar trial of AIP in 20 patients with atopic dermatitis. In addition to assessing changes in skin symptoms, we will also be tracking how the diet changes the gut microbiome and intestinal permeability! I’m excited to learn more about how AIP works on the molecular level.
AIP quick start guide
So, how to get started? The simple answer is to remove the foods listed above and focus on nutrient-dense foods like meat, broth, vegetables and fermented foods. For more detailed instructions, here are a few of my favorite resources for getting started with AIP:
The Autoimmune Protocol – Sarah Ballantyne’s quick start guide to AIP
“AIP for you and me” – an AIP Facebook support group
The Paleo Approach – Sarah Ballantyne’s book (and matching cookbook)
SAD to AIP in six – Angie Alt’s program used in the AIP for IBD study
And here are a few of my favorite AIP-friendly recipes:
That’s all for now! If you enjoyed this article, be sure to subscribe. You can also click here to learn more about working with me one-on-one.
Sources:
- Flint, H. J., Scott, K. P., Louis, P. & Duncan, S. H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589 (2012).
- Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M. & Owen, L. J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191 (2015).
- Jangi, S. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016).
- He, Z., Shao, T., Li, H., Xie, Z. & Wen, C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 8, 64 (2016).
- Giongo, A. et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5, 82–91 (2011).
- Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
- Vojdani, A. & Tarash, I. Cross-Reaction between Gliadin and Different Food and Tissue Antigens. Food Nutr. Sci. 04, 20 (2013).
- Ventura, M. T. et al. Intestinal permeability in patients with adverse reactions to food. Dig. Liver Dis. 38, 732–736 (2006).
- Visser Jeroen, Rozing Jan, Sapone Anna, Lammers Karen & Fasano Alessio. Tight Junctions, Intestinal Permeability, and Autoimmunity. Ann. N. Y. Acad. Sci. 1165, 195–205 (2009).
- König, J. et al. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 7, e196 (2016).
- Vaarala, O., Atkinson, M. A. & Neu, J. The “Perfect Storm” for Type 1 Diabetes: The Complex Interplay Between Intestinal Microbiota, Gut Permeability, and Mucosal Immunity. Diabetes 57, 2555–2562 (2008).
- Ma, T. Y. et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G367-376 (2004).
- Konijeti, G. G. et al. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 23, 2054–2060 (2017).
- Lammers, K. M. et al. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 135, 194-204.e3 (2008).
- Drago, S. et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 41, 408–419 (2006).
- Thomas, K. E., Sapone, A., Fasano, A. & Vogel, S. N. Gliadin Stimulation of Murine Macrophage Inflammatory Gene Expression and Intestinal Permeability Are MyD88-Dependent: Role of the Innate Immune Response in Celiac Disease. J. Immunol. 176, 2512–2521 (2006).
- Francis, G., Kerem, Z., Makkar, H. P. S. & Becker, K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 88, 587–605 (2002).
- Gee, J. M. et al. Effects of saponins and glycoalkaloids on the permeability and viability of mammalian intestinal cells and on the integrity of tissue preparations in vitro. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 10, 117–128 (1996).
- Patel, B. et al. Potato glycoalkaloids adversely affect intestinal permeability and aggravate inflammatory bowel disease. Inflamm. Bowel Dis. 8, 340–346 (2002).
- Jönsson, T. et al. A Paleolithic diet confers higher insulin sensitivity, lower C-reactive protein and lower blood pressure than a cereal-based diet in domestic pigs. Nutr. Metab. 3, 39 (2006).
- Jensen-Jarolim, E. et al. Hot spices influence permeability of human intestinal epithelial monolayers. J. Nutr. 128, 577–581 (1998).
- Monetini, L. et al. Antibodies to Bovine Beta-Casein in Diabetes and Other Autoimmune Diseases. Horm. Metab. Res. 34, 455–459 (2002).
- Heine, R. G., Laske, N. & Hill, D. J. The diagnosis and management of egg allergy. Curr. Allergy Asthma Rep. 6, 145–152 (2006).
- Purohit, V. et al. Alcohol, Intestinal Bacterial Growth, Intestinal Permeability to Endotoxin, and Medical Consequences. Alcohol Fayettev. N 42, 349–361 (2008).
- Patel, S. et al. Alcohol and the Intestine. Biomolecules 5, 2573–2588 (2015).
- Roux, K. H., Teuber, S. S. & Sathe, S. K. Tree Nut Allergens. Int. Arch. Allergy Immunol. 131, 234–244 (2003).
- Lerner, A. & Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 14, 479–489 (2015).
- Graf, D. et al. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 26, 26164 (2015).
- Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
- Martínez-Montiel, M. et al. Pharmacologic therapy for inflammatory bowel disease refractory to steroids. Clin. Exp. Gastroenterol. 8, 257–269 (2015).

[…] foods are removed from the diet, in an effort to determine which may be causing their symptoms. The Autoimmune Paleo Diet removes most of the common plant compounds mentioned above and is regarded as a more complete […]
I would be interested to know if you remain convinced about the rationale for the exclusions of the various categories? It can all seem a little cranky. I believe there is now evidence of human grain cultivation and consumption long before the advent of agriculture. And surely nuts would have been a component of human diets forever, since they are an easy source of protein and fats. Maybe it is a quantity thing? I know that many people do well on the diet, but it would be good to have stronger MECHANISTIC evidence/rationale, since people also often run into roadblocks, and then have no well-motivated criterion by which to choose how to act.